
Protecting Patients,
Not Trying Patience.
Your IRB, Simplified

Navigate the Path to Study Startup, Faster
Partner with Univo IRB for a forward-thinking approach, supportive solutions, and modern technology that make IRB review simple and helpful.
Because our goal is to protect your patients, not try your patience.
Unifying Patients & Research
Univo IRB is the only institutional review board (IRB) focused on enhancing patient participation, education, and advocacy. When we make clinical trials better for patients, they get easier for researchers, and we all benefit from more treatment options.
Your Single IRB
IRBs have become too big, unfocused, and bureaucratic, slowing down your clinical trial and distracting from their core responsibility to protect the rights and welfare of research participants.
It doesn’t have to be so complicated! To get your study to the next milestone safely and quickly, you need your IRB, simplified.
Univo is led by independent IRB and clinical operations experts that are on a mission to unify patients with research. We know the reality of running clinical trials and offer our support where it’s most helpful.

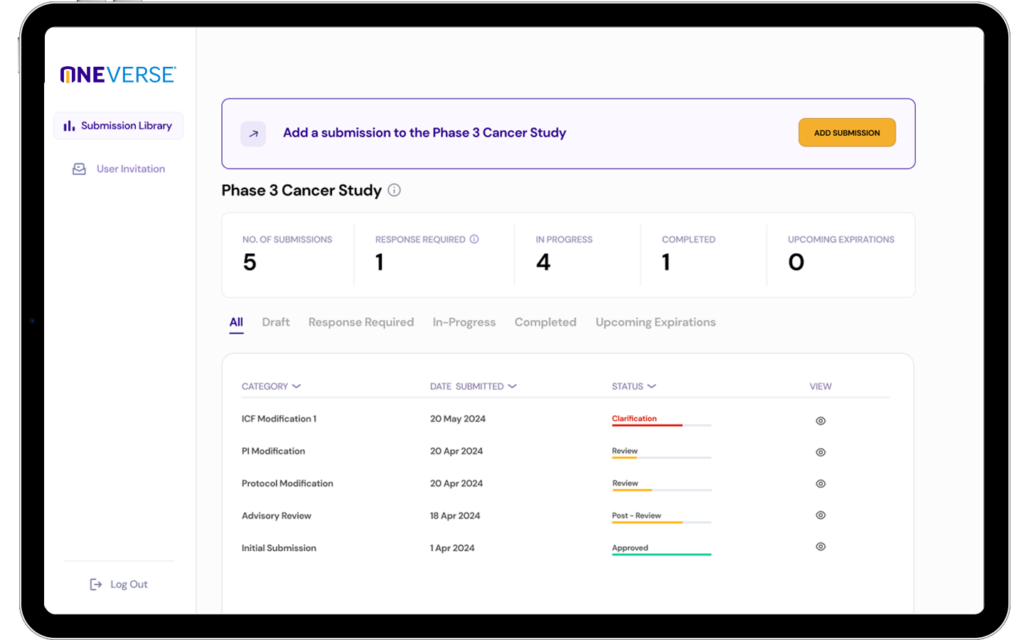
OneVerse®
OneVerse is the only purpose-built IRB platform for central IRB review. Unlike dated systems that have been modified from decades-old, single-site research technology, it enables the highest efficiency of virtual workforces so you can enjoy superior service.
- See all of your submissions and action items in one place
- Customized to enhance your experience and meet your needs
- Auto-population — never retype the same information again
- Instant access to searchable resources and support
- Easily review approved documentation, amendments, and updates




